Adaptive Population Enrichment (APE)
Overview
In summary:
- The goal of APE is to simultaneously evaluate the effects of study treatment both in the broad target population and subpopulations of interest with sufficient statistical power in a single trial;
- APE is appropriate when there is considerable uncertainty about whether there are subpopulations of patients within the broad target population that would benefit more from the treatment than others;
- The trial starts by enrolling a broad patient population, but with the option to restrict future recruitment to selected subpopulations that are indicating treatment benefits, based on interim results;
- The entire trial can be stopped early if the study treatment is unlikely to benefit any subpopulation, or if there is overwhelming evidence of benefit in all subpopulations to reach conclusions;
- The criteria for selecting/dropping subpopulations or stopping the trial early at interim analyses must be prespecified;
- Subpopulations of interest must be known and defined in advance such as based on patient characteristics or a biomarker.
VIDEO BEING UPLOADED
Motivation
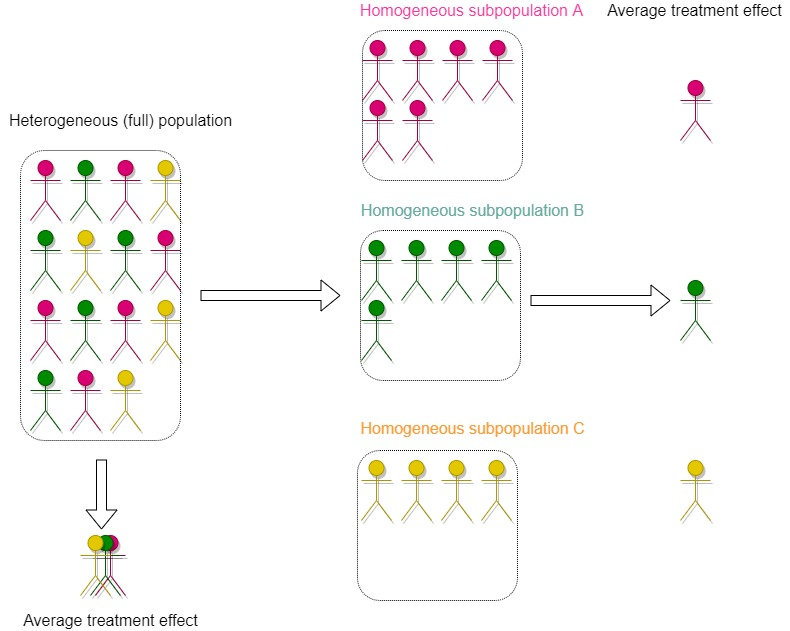
Before a trial begins, researchers must decide on a target population that comprises patients that are expected to benefit from the study treatment. The treatment may have small or even no impact in some subgroups of patients within the target population, and in practice, there are often considerable uncertainties around the target population to study 1. As such, researchers are faced with a challenging decision as to whether to restrict the inclusion criteria to only enrol a specific (homogeneous) subset of patients or to broaden inclusion criteria to include a more diverse (and thereby heterogeneous) patient population (see Figure 1 illustration). Selecting homogeneous patients limits the generalisability of trial results to influence practice in untested populations – so unanswered questions will remain after the trial 2. On the contrary, although the inclusion of heterogeneous patients allows researchers to answer questions relevant to a broader population, potentially effective treatments for a subset of the broader population may fail to show benefits because trial patients unlikely to benefit have also been included in the trial (see 3 for discussion).
Traditionally, clinical trials are often conducted with an expectation that if a treatment is truly beneficial, it should, on average, benefit a broad target population. However, this “one-size-fits-all” notion is not always correct. Some patient subgroups may show greater treatment benefit than others, possibly due to specific characteristics (e.g., age, sex, previous treatments given), differences in underlying patient risks, or disease severity. For example, trastuzumab is a targeted cancer drug that has been shown to be effective in treating patients with breast or stomach tumours expressing large amounts of a protein called human epidermal growth factor receptor 2 (HER2) rather than all patients with breast or stomach tumours. As a result, if a treatment works only in certain patient subgroups, finding a truly effective treatment in a broader group of patients can be challenging because the overall average effect is diluted.
An adaptive population enrichment (APE) design addresses this problem by allowing researchers to learn from interim efficacy results and use this information to choose a more specific patient subpopulation likely to benefit from an effective treatment. Figure 2 presents multiple research questions that can be simultaneously answered in a single trial using an APE design.
In summary
An APE design allows researchers to find the right patient groups who may benefit from the study treatment by changing the eligibility criteria during the trial, based on predefined rules at interim analyses. As such, trial resources are directed towards selected subpopulations that are showing greater treatment benefit at interim analyses. Most importantly, the promising subpopulations are enriched to ensure that there is adequate statistical power to evaluate treatment effects in each selected subpopulation.
In contrast
In a fixed design trial, the primary objective is to evaluate the effects of study treatment in the entire target population. The evaluation of treatment effects in prespecified subgroups (subpopulations) is often set aside as an exploratory objective because the trial usually does not have enough power to detect clinically meaningful treatment effects in these subpopulations (see 4 for discussion). As such, signals of treatment efficacy in certain subpopulations are primarily for hypothesis generation to inform future research. For instance, exploratory results may suggest that further trials focusing on enrolling these promising subpopulations are needed. This would require more resources, time, and trial participants to conduct subsequent independent trials enrolling each promising subpopulation, rather than running a single APE trial with a shared comparator group.
Of note, using interim analyses to select promising subpopulations pose a multiple testing problem which should be accounted for when designing a APE trial and the trial is designed with adequate statistical power to investigate treatment effects in selected subpopulations (see underpinning statistical methods).
References
1. Fisher et al. Stochastic optimization of adaptive enrichment designs for two subpopulations. J Biopharm Stat. 2018;28(5):966-982.
2. Freidlin et al. Biomarker-adaptive clinical trial designs. Pharmacogenomics. 2010;11(12):1679-1682.
3. Green et al. Enrichment strategies in pediatric drug development: An analysis of trials submitted to the US Food and Drug Administration. Clin Pharmacol Ther. 2018;104(5):983-988.
4. Alosh et al. Tutorial on statistical considerations on subgroup analysis in confirmatory clinical trials. Stat Med. 2017;36(8):1334-1360.
2. Freidlin et al. Biomarker-adaptive clinical trial designs. Pharmacogenomics. 2010;11(12):1679-1682.
3. Green et al. Enrichment strategies in pediatric drug development: An analysis of trials submitted to the US Food and Drug Administration. Clin Pharmacol Ther. 2018;104(5):983-988.
4. Alosh et al. Tutorial on statistical considerations on subgroup analysis in confirmatory clinical trials. Stat Med. 2017;36(8):1334-1360.
In context
One reason attributed to treatment failures in phase 3 trials in certain disease areas (e.g. in oncology) is the inability to identify appropriate target populations in which the study treatment is likely to show benefit 1. In paediatric trials, the use of generic enrichment strategies was associated with increased regulatory approvals by an average of 78% (across therapeutic areas) 2. The strategies were used across 16 therapeutic areas such as pulmonary, psychiatry, antiviral, anti-infectives, and neurology. These approaches are discussed in other disease areas (e.g. sepsis) 3. I-SPY 2 4, 5, 6 is a trial that used an APE design successfully to find effective treatments for specific biomarker-defined subpopulations (tumour type) where more patients were allocated to subgroups showing greater benefit (response-adaptive randomisation, RAR design) with options to graduate efficacious treatments early for confirmatory testing or to drop futile treatments 4, 6.
References
1. Mehta et al. Biomarker-driven population enrichment for adaptive oncology trials with time to event endpoints. Stat Med. 2014;33(26):4515-4531.
2. Green et al. Enrichment strategies in pediatric drug development: An analysis of trials submitted to the US Food and Drug Administration. Clin Pharmacol Ther. 2018;104(5):983-988.
3. Stanski et al. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol. 2020;16(1):20-31.
4. Park et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375(1):11-22.
5. Nanda et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: An analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6(5):676-684.
6. Barker et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86(1):97-100.
2. Green et al. Enrichment strategies in pediatric drug development: An analysis of trials submitted to the US Food and Drug Administration. Clin Pharmacol Ther. 2018;104(5):983-988.
3. Stanski et al. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol. 2020;16(1):20-31.
4. Park et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375(1):11-22.
5. Nanda et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: An analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6(5):676-684.
6. Barker et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86(1):97-100.
Illustrative example: not a real trial
People with multiple sclerosis frequently report depression or low mood 1. Any intervention aiming to improve the quality of life in this patient group may benefit from being tailored to incorporate and address the specific disease symptoms. For this example, let us assume we want to develop and trial a behavioural intervention specific to multiple sclerosis patients.
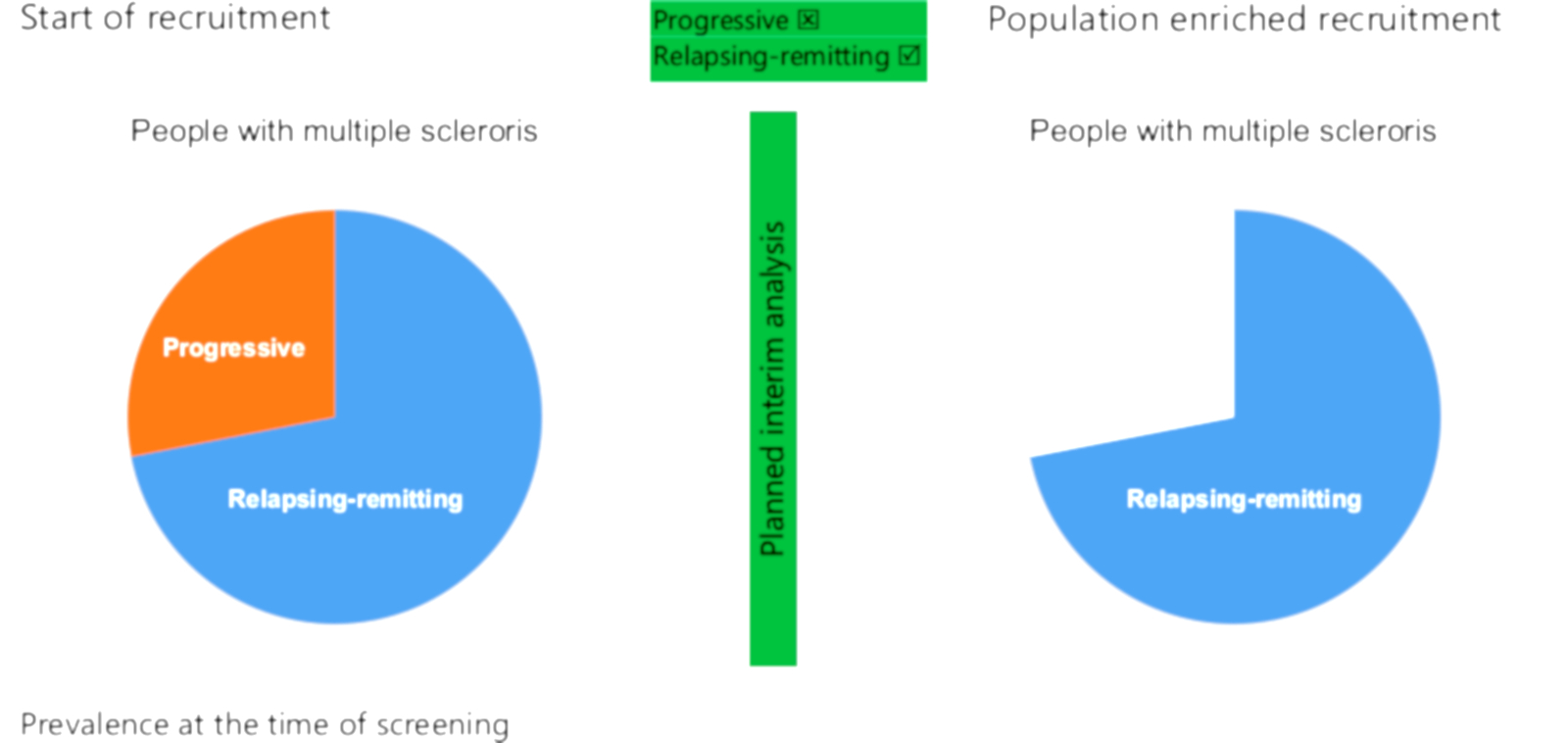
Who should our target population be? The natural choice is “all patients with multiple sclerosis”. But multiple sclerosis patients are themselves heterogeneous. One key characteristic is whether the disease is “relapsing-remitting” (i.e. where symptomatic attacks come in phases but eventually subside), or “progressive” (i.e. where the symptoms do not subside after onset). It is reasonable to suggest these two multiple sclerosis subpopulations may respond differently to any behavioural intervention.
An APE design may be useful here. The trial could initially recruit all multiple sclerosis patients but build in an interim analysis that assesses whether the intervention appears beneficial to both subpopulations. If it doesn’t appear to work well in one, future recruitment can be limited to the other such as shown in Figure 3. This design has some drawbacks. The subpopulations need to be defined before the trial beings, something which is seldom easy. Patients with multiple sclerosis can be categorised in many ways aside from the above – indeed, “relapsing-remitting” itself can be further subdivided, as can “progressive”. Moreover, more subpopulations mean a smaller number of potentially available participants, which may make trials infeasible for uncommon diseases such as multiple sclerosis.
References
1. Siegert et al. Depression in multiple sclerosis: A review. J Neurol Neurosurg Psychiatry. 2005;76(4):469-475.